Research interests
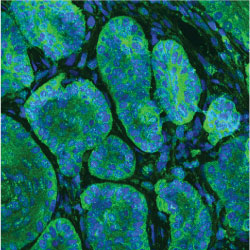
Cancer initiation and progression
Mutations that activate the Hedgehog pathway drive growth of a variety of cancers including basal cell carcinoma (BCC) and medulloblastoma, accounting for up to 25% of all human cancer deaths. Despite the critical nature of Hedgehog signaling during development, how Hedgehog mediates tumor initiation, growth, regression, and drug resistance remains poorly understood. For instance, use of drugs that target the Hedgehog pathway GPCR Smoothened are effective in treating advanced or metastatic BCC, however over 50% of advanced tumors harbor innate resistance and over 20% of tumors that initially respond to drug acquire resistance each year, illustrating the need for additional targets for therapy. Our lab has identified atypical Protein Kinase C (aPKC) as a novel Hedgehog target gene and activator of Hedgehog signaling. aPKC forms a positive feedback loop by phosphorylating and activating the transcription factor GLI1, resulting in an increase in DNA binding and transcriptional activity. Interestingly, sensitive and drug resistant BCCs magnify aPKC activity to drive high levels of pathway activation and therapeutic use of aPKC or GLI inhibitors selectively suppress Hedgehog signaling and tumor growth, suggesting the use of aPKC or GLI antagonists as viable options to treat drug-resistant tumors. Projects in the lab include defining the drivers of tumor regression, identifying and therapeutically targeting novel kinase-substrate interactions that drive tumor growth, understanding how recurrent mutations translate to functional consequences, and delineating how tumor cells interact with their environment.
Immune regulation of skin homeostasis and skin cancer
Phenotypically normal aged skin is often highly mutated with alterations in oncogenes and tumor suppressors that would induce tumor growth in mice. How skin prevents most tumor clones from growing past tissue boundaries remains unknown. We are currently using a BCC mouse model to define how the immune system monitors tumor clones and elicits an immune response to eradicate oncogenic outgrowths. When large BCC outgrowths do appear in aged skin, response to immunotherapy is highly variable. Traditionally thought to be a “cold” cancer that does not readily incite an immune response, immune-BCC interactions do occur but with far less frequency than other skin cancers. Using single cell RNA-sequencing approaches, analysis cell-cell communication, and mathematical modeling in late-stage tumors, we discovered potential biomarkers that may predict response to checkpoint immunotherapy. Projects in the lab include characterizing the immunogenicity of BCC, uncovering ways to therapeutically increase tumor immunogenicity, understanding how tumor-derived signals suppress the immune system, and defining how the immune system regulates tumor regression.
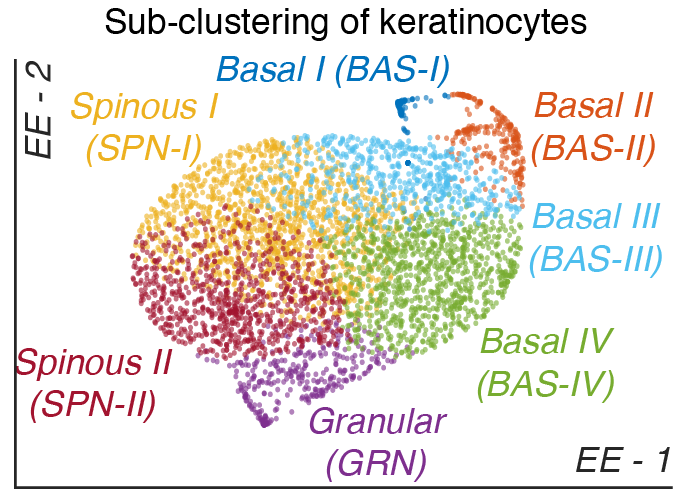
Skin stem cell fate specification
Stem cells give rise to many complex tissues such as the skin and hair follicle by controlling cell fate specification. How epidermal stem cells choose their fate remains unclear. Our lab has identified robust heterogeniety in the basal stem cells of human skin through single cell RNA-sequencing that changes they way we appreciate how the human epidermis develops. We have identified four spatially distinct epidermal stem cell subpopulations that contribute to epidermal developoment and homeostasis. Projects in the lab include defining how each epidermal stem cell subpopulation are related by lineage, how they communicate, how they pattern the skin, and which subpopulation preferentially gives rise to hyperproliferative skin disorders such as psoriasis and basal cell carcinoma.
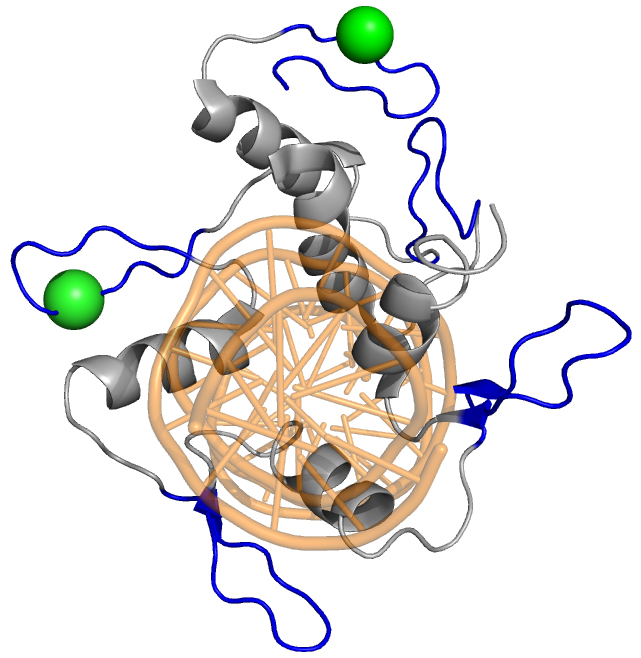
Transcription factor regulation
Post-translational modifications drive the function of transcription factors by serving as scaffolds, binary switches, and regulating transport. The master polarity regulator aPKC, which is found in virtually all polarized systems and serves to regulate cell polarity and fate specification, partly serves to regulate transcription factor function. Our lab has found that aPKC phosphorylates the C2H2 zinc finger domain of GLI1 to promote DNA binding and transcriptional activity, an exceedingly rare event that serves as a positive signal to promote transcription factor function. Projects in the lab include exploring how phosphorylation positively and negatively regulates transcription factor activity, investigating how cancers co-opt this signaling process to drive tumor growth, and designing synthetically regulatable transcription factor cassettes to treat disease.